UK Issues Its First License for Mitochondrial Replacement to Produce Healthy Babies
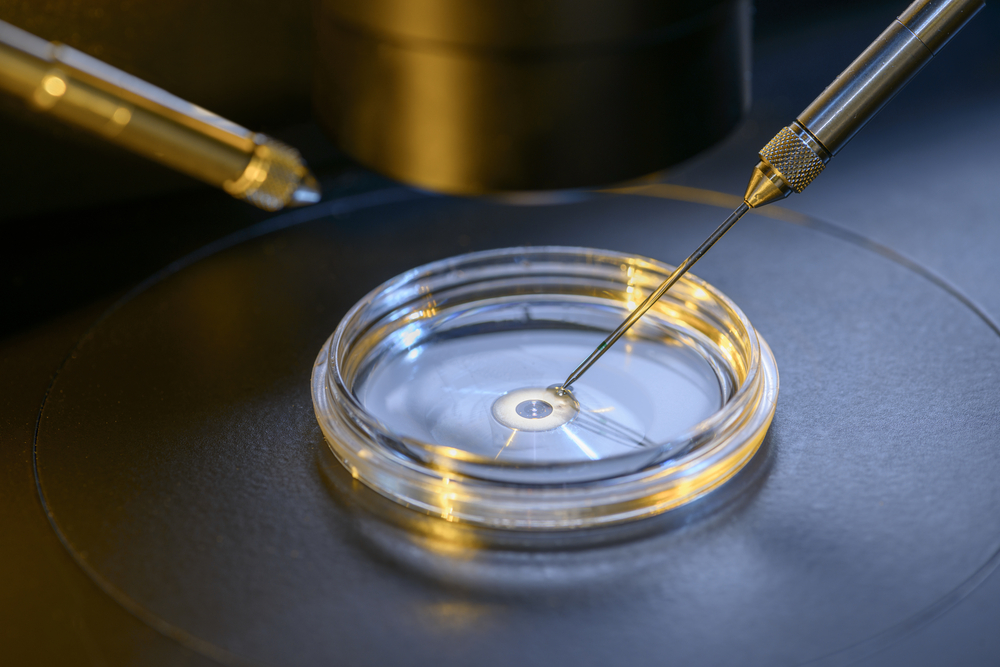
The United Kingdom has issued its first license for research that involves women with mitochondria disease receiving donated egg components so they can produce healthy children.
Newcastle University researchers will use the U.K. Human Fertilisation and Embryology Authority (HFEA) license to study mitochondrial replacement in 25 women a year for five years. The authority and research team must agree on which patients to include in the study, and what treatments they will receive, before the clinical trial can begin.
Mitochondrial replacement is also known as mitochondrial IVF. It involves transferring the DNA-containing nucleus of an egg with faulty mitochondria to a donor with healthy mitochondria whose egg-cell nucleus has been removed. The procedure gives women with mitochondrial disease a chance to have healthy children.
“HFEA has approved the first application by Newcastle Fertility at Life for the use of mitochondrial donation to treat patients,” Sally Cheshire, the chair of HFEA, said in a press release. “This significant decision represents the culmination of many years’ hard work by researchers, clinical experts, and regulators, who collectively paved the way for Parliament to change the law in 2015 to permit the use of such techniques. Patients will now be able to apply individually to the HFEA to undergo mitochondrial donation treatment at Newcastle, which will be life-changing for them, as they seek to avoid passing on serious genetic diseases to future generations.”
Professor Sir Doug Turnbull and colleagues at the Wellcome Trust Center for Mitochondrial Research in Newcastle pioneered mitochondrial replacement 10 years ago.
“Today’s decision means that affected couples in the UK who have dreamt of having a baby free of mitochondrial disease will have an option open to them for the first time,” the director of the Wellcome Trust, Professor Jeremy Farrar, said in a news release.
Mitochondrial disease is the name scientists have given to a group of diseases caused by genetic mutations in mitochondria DNA. Transfering the nucleus of an egg, which contains almost all of the cell’s genetic information, to an egg cell with healthy mitochondria could prevent the disease from being passed to future generations.
The license will enable Turnbull and his team to check the safety of the technique so the service can be offered across the U.K.
Currently, the HFEA must approve mitochondrial replacement in every patient and every clinic case by case. It allows the treatment only in cases where a child is at high risk of developing mitochondrial disease.
“Mitochondria diseases can be devastating for families affected, and this is a momentous day for patients who have tirelessly campaigned for this decision,” Turnbull said.
Although the U.K. is the first country to approve the technique, U.S. researchers have used it in Mexico, where it led to the birth of a healthy baby.
At the moment, legislation prohibits the technique’s use in the United States.






