Nucleoside Therapy Explained: Why Doctors Hesitate to Treat Charlie Gard

[Updated 2:00 pm ET — According to multiple sources, Charlie Gard’s parents have indicated that they will withdraw their legal bid in the United Kingdom to allow their son to seek experimental treatment, citing recent MRI scans that clearly show that the therapy would likely be ineffective and could lead to pain and suffering.]
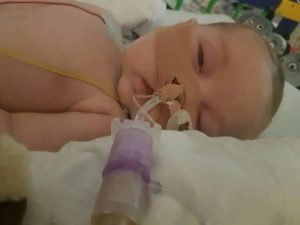
With Charlie Gard — a child born with a fatal mitochondrial disease — making headlines as his parents wage a life-or-death fight with the U.K. jurisdiction, his proposed treatment, called nucleoside therapy, also has found itself in the spotlight.
Although The Telegraph reported that the family has been granted permanent resident status in the U.S. so Charlie can receive the experimental treatment, physicians and judges are still debating whether he will receive receive it.
Charlie’s physicians at the Great Ormond Street Hospital (GOSH), considered to be highly experienced in treating children with mitochondrial diseases, believe the treatment will not help 11-month-old Charlie, but rather prolong his suffering, as Mitochondrial Disease News recently reported. They argue he should be allowed to die with dignity.
But his parents, along with experts from the U.S. and Italy, among others, contend there is a chance that the highly experimental treatment might improve Charlie’s condition. He is now unable to move, breathe for himself, and is thought to have extensive brain damage.
But what is this treatment that experts in the field fail to agree would be of benefit to the child with a severe mitochondrial disease?
Life’s building blocks
Our bodies’ cells hold the entire genetic code to make sure each cell does what it is supposed to do. Our DNA hold genes that, by being switched on or off, allow each cell to perform specialized functions.
But DNA can be found outside the nucleus of a cell. Mitochondria, converting nutrients to energy, hold their own DNA, needed to control the processes of cellular energy metabolism.
Each time mitochondria divide to produce new energy factories they needs to make new DNA. The same is true when DNA becomes damaged.
DNA is made up of four types of letters — G, C, A, and T. These are called nucleotides.
Charlie Gard’s mutation affects a gene called RRM2B. The gene is involved in making the four DNA letters inside mitochondria. The inability to produce the DNA building blocks causes mitochondrial depletion syndrome, the disease with which Charlie was born.
Without DNA and functioning energy-making processes, the body quickly loses its ability to function. Muscles and the brain are affected in early disease stages, because they require more energy than other organs.
Nucleosides to nucleotides
The therapy that some argue should be tried in Charlie’s case is virtually a supplementation of the missing building blocks. But, instead of giving a child nucleotides, researchers have found that the compounds turning into the four DNA letters, called nucleosides, are better to use for treatment.
When given orally, they pass the gut without being degraded, and find their way to the body’s millions of mitochondria. Since the rest of the machinery is intact, they are converted to nucleotides and can be used to build DNA, restoring the energy-making processes.
Experimental treatment
Nucleoside therapy was developed by Michio Hirano, MD, a neurologist and neuromuscular disease expert at Columbia University. While nucleoside treatment has not been evaluated in clinical trials, it has been used in 18 children with another form of mitochondrial depletion syndrome, according to an article in Scientific American.
The 18 treated patients all have mitochondrial depletion syndrome caused by mutations in a gene called TK2. In similarity with RRM2B, it is involved in making DNA building blocks.
Researchers have not publicly disclosed how the treatment has worked for the majority of these patients. But, as we previously reported, the parents of an American boy, Arthur and Olga Estopinan, have shared the story of their son’s treatment.
In similarity to Charlie, he could not move his fingers or toes or breathe unassisted. Now 6 years old, Arturito is steadily improving, and now can stand and communicate in a basic way, although he still requires a ventilator and around-the-clock care.
So, why not Charlie?
Charlie’s type of mitochondrial depletion syndrome is utterly rare. Researchers have encountered only 15 other children with the disease caused by mutations in the RRM2B gene. So, while there is some evidence of how the nucleoside treatment works in both animal models and patients with TK2 mutations, there is none for Charlie’s form of the disease — not even from mice.
Moreover, GOSH physicians claim Charlie’s brain damage is too severe to make any treatment meaningful. The damage is irreversible, they argue, and although his mitochondria will start working he will never become a functioning child.
But Charlie’s parents do not agree, and Hirano himself examined the boy and analyzed new brain scans on Monday in the search for evidence that the treatment might work.
Earlier court hearings also revealed that experts differ in how they assess the likelihood the treatment will work in Charlie’s case. The U.K. has a system in which the decision of removing life-support is based on court rulings, differing from the common practice in the U.S. to let parents decide, according to an BBC News article.
And even if a clinical trial of the treatment is on the horizon, it is unlikely that children with RRM2B mutations will be part of that study. For future children born with the more common TK2 type of mitochondrial depletion, this research might change the course of their lives.