University Researchers Lead $1.4M Defense Project to Study Origin of Leigh’s Disease
 Researchers at the University of Arkansas’ bioenergetics lab at its Fayetteville campus will lead a team of collaborators in a study funded by a $1.4 million grant from the U.S. Department of Defense.
Researchers at the University of Arkansas’ bioenergetics lab at its Fayetteville campus will lead a team of collaborators in a study funded by a $1.4 million grant from the U.S. Department of Defense.
Its main goal is to investigate the origin of Leigh’s disease, a rare and incurable disorder that affects the central nervous system.
The disease, also known as Leigh syndrome, is described by the National Institute of Neurological Disorders and Stroke (NINDS) as an inherited neurometabolic disorder that typically manifests in infants between the ages of 3 months and 2 years. Very occasionally it develops in teenagers and adults, and can be caused by mutations in mitochondrial DNA or by a deficiency in an enzyme called pyruvate dehydrogenase.
 According to the National Organization for Rare Disorders (NORD), a nonprofit advocacy and research fundraising organization dedicated to providing support for individuals with rare diseases.
According to the National Organization for Rare Disorders (NORD), a nonprofit advocacy and research fundraising organization dedicated to providing support for individuals with rare diseases.
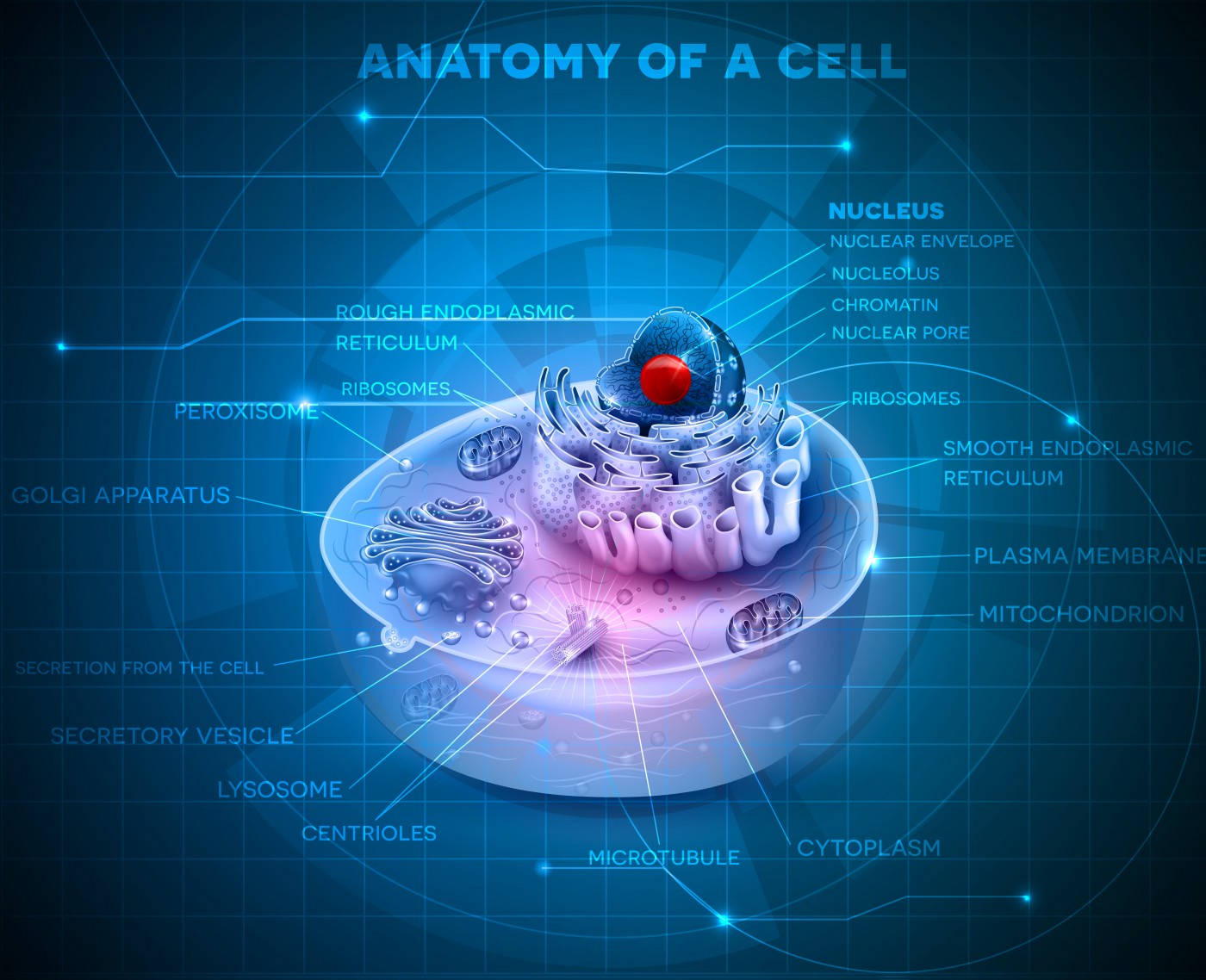
NINDS explains that in Leigh’s disease, genetic mutations in mitochondrial DNA interfere with the energy sources that power cells in an area of the brain that controls motor movement. Genetic mutations in mitochondrial DNA result in a chronic lack of energy in these cells, in turn affecting the central nervous system and causing progressive degeneration of motor functions.
Mitochondria is intimately involved in the active function of many key tissues that include the brain, heart, and skeletal muscles. The current prognosis for individuals with Leigh’s disease is poor.
Diagnosing Leigh’s disease may be confirmed by a thorough clinical evaluation and a variety of tests, particularly advanced imaging techniques such as MRI or computed tomography (CT) scans of the brain. There are no proven therapies for Leigh’s disease, and available treatments only improve systems and slow the progression of the disease.
According to NINDS, the most common treatment is thiamine or vitamin B1, and oral sodium bicarbonate or sodium citrate may also be prescribed to manage lactic acidosis. In individuals who have the X-linked form of Leigh’s disease, a high-fat, low-carbohydrate diet may be recommended.
 “The goal of the three-year study is to provide a better understanding of the emergence of the mitochondrial mutation that causes the disease,” Shilpa Iyer, an assistant professor in the J. William Fulbright College of Arts and Science at the University of Arkansas who is leading the study, said in a press release.
“The goal of the three-year study is to provide a better understanding of the emergence of the mitochondrial mutation that causes the disease,” Shilpa Iyer, an assistant professor in the J. William Fulbright College of Arts and Science at the University of Arkansas who is leading the study, said in a press release.
Iyer’s bioenergetics research program broadly focuses on understanding the genetics and developing therapies for childhood and age-related mitochondrial diseases. The U of A researchers are generating patient-specific stem cells to facilitate therapies and understand the cause and progression of mitochondrial diseases.
The research has military relevance in that mitochondrial function directly affects the energy levels and functional capabilities of returning combat veterans.
If this Department of Defense-funded project is successful, the researchers will generate clinical-grade, patient-specific stem cells for drug discovery and transplant therapies to be used with Leigh’s syndrome patients. Skin cells from a patient diagnosed with mitochondrial and other energy-deficiency diseases are being genetically reprogrammed into stem cells at Iyer‘s bioenergetics lab, then transformed into neurons and muscle cells.
Additional studies will identify the many steps in cellular bioenergetics that will help in understanding how stem cells are transformed into neurons and muscle cells in normal and diseased patients. The team will sample skin cells taken from adults suffering from Leigh’s disease and re-engineer them into a state identical to that of an embryonic stem cell.
“We are mimicking the earliest stages of human development to trace how the mitochondria mutation is transmitted in each cell,” Iyer said. “In essence, we are re-creating the disease in the lab. We are trying to provide hope for people who are very sick.”
Iyer was also recently awarded a $236,946 transfer grant from the National Institutes of Health to fund a similar mitochondrial research project she started at her previous institution.
“Our multidisciplinary research team is using advanced genetic techniques to better understand how and when mutations in the mitochondrial genome develop and cause these diseases,” Iyer said.
 The researchers include Raj Rao, head of the Department of Biomedical Engineering in the College of Engineering at the U of A; researchers at the Hunter Holmes McGuire VA Medical Center in Richmond, Virginia; and at the University of Georgia.
The researchers include Raj Rao, head of the Department of Biomedical Engineering in the College of Engineering at the U of A; researchers at the Hunter Holmes McGuire VA Medical Center in Richmond, Virginia; and at the University of Georgia.






